J Antibiot (Tokyo). 2021 Dec 21;75(2):60–71. doi: 10.1038/s41429-021-00491-6
The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review
Asiya Kamber Zaidi 1,✉, Puya Dehgani-Mobaraki 1
- Author information
- Article notes
- Copyright and License information
PMCID: PMC8688140 PMID: 34931048
See the original article “RETRACTED ARTICLE: The mechanisms of action of Ivermectin against SARS-CoV-2: An evidence-based clinical review article” on page 122.
Abstract
Considering the urgency of the ongoing COVID-19 pandemic, detection of new mutant strains and potential re-emergence of novel coronaviruses, repurposing of drugs such as ivermectin could be worthy of attention. This review article aims to discuss the probable mechanisms of action of ivermectin against SARS-CoV-2 by summarizing the available literature over the years. A schematic of the key cellular and biomolecular interactions between ivermectin, host cell, and SARS-CoV-2 in COVID-19 pathogenesis and prevention of complications has been proposed.
Subject terms: Viral infection, Receptor pharmacology
Introduction
A relatively recent surge in zoonotic diseases has been noted over the past few decades. Several reasons could be responsible for this “spill-over” of disease-causing agents from animals to humans. These include an exponential rise in the global population causing man to encroach new ecological habitats in search of space, food, and resources as well as improved opportunities for rampant wildlife trade causing interspecies pathogen jumps. The 1980s was known for HIV/AIDS crisis that originated from the great apes, while the avian flu pandemic in 2004–07 came from the birds. The pigs led to the swine flu pandemic in 2009 and bats were the original hosts of Ebola, severe acute respiratory syndrome (SARS), Middle Eastern respiratory syndrome, and probably SARS coronavirus 2 (SARS-CoV-2) outbreak as well.
COVID-19 has already caused millions of deaths worldwide and has paralyzed not only the world’s healthcare system but also the political and economic relations between countries [1]. The fact that the SARS-CoV-2 virus has been thought to have originated from wildlife and may have “jumped” into humans, not only highlights future risks from animal-borne diseases but also provides an important clue to its resolution. In such a scenario, where this “jump” has been made from animal to human, it seems only logical to review a drug that has worked efficiently against a disease-causing agent and is available in a form that is safe for human consumption since the early 1980s.
Ivermectin belongs to a group of avermectins, which is a group of 16 membered macrocyclic lactone compounds discovered at the Japanese Kitasato Institute in 1967 during actinomycetes cultures with Streptomyces avermitilis [2]. This drug radically lowered the incidence of river blindness and lymphatic filariasis and was discovered and developed by William C. Campbell and Satoshi Ōmura for which they received the Nobel Prize in Physiology or Medicine in 2015 [3, 4]. Ivermectin is enlisted in the World Health Organization’s Model List of Essential Medicines [5].
Drug repurposing, drug redirecting, or drug reprofiling is defined as the identification of novel uses for existing drugs. The development risks, costs as well as safety-related failure, are reduced with this approach since these drugs have a well-established formulation development, in vitro and in vivo screening, as well as pharmacokinetic and pharmacodynamic profiles. Moreover, the first clinical trial phases of many such drugs have been completed and can be bypassed to reduce several years of development. Therefore, drug repurposing has the potential to reduce the time frame for the whole process by up to 3–12 years and carries great potential [6].
Although several drugs received emergency use authorization for COVID-19 treatment with unsatisfactory supportive data, ivermectin, on the other hand, has been sidelined. Nevertheless, many countries adopted ivermectin as one of the first-line treatment options for COVID-19.
With the ongoing vaccine roll-out programs in full swing across the globe, the longevity of the immunity offered by these vaccines or their role in offering protection against new mutant strains is still a matter of debate. Thus, the search for new, effective antivirals continues.
Several doctor-initiated clinical trial protocols that aimed to evaluate outcomes, such as reduction in mortality figures, shortened length of intensive care unit stay and/or hospital stay, and elimination of the virus with ivermectin use have been registered at the US ClinicalTrials.gov [7]. Controlled clinical trials using ivermectin are underway, including one being conducted by the National Institutes of Health (ACTIV-6) [ClinicalTrials.gov Identifier: NCT04885530] in the USA and a second in the UK (PRINCIPLE) [ISRCTN registry: ISRCTN86534580] [8, 9].
Ivermectin has rapid oral absorption, high liposolubility, is widely distributed in the body, metabolized in the liver (cytochrome P450 system), and excreted almost exclusively in feces [4]. Following a standard oral dose in healthy humans, it reaches peak plasma levels at 3.4–5 h, and plasma half-life has been reported to be 12–66 h [10]. Despite its widespread use, there are relatively few studies on the pharmacokinetics of ivermectin in humans [11]. Ivermectin binds strongly to plasma proteins in healthy subjects (93.2%) [12]. Such an “avid binding” can be beneficial when administered in countries where malnutrition and hypoalbuminemia are common, leading to increased availability of “free fraction” of ivermectin [4]. Hypoalbuminemia is a frequent finding in patients with COVID‐19 and it also appears to be linked to the severity of lung injury [13]. Therefore, ivermectin could have sufficient bioavailability when used in such a setting.
This article aims to discuss the probable mechanisms of action by summarizing the in vitro and in vivo evidence demonstrating the role of ivermectin in COVID-19 based on the available literature over the years (Table 1). A schematic of the key cellular and biomolecular interactions between ivermectin, host cell, and SARS-CoV-2 in COVID-19 pathogenesis and prevention of complications has been proposed (Fig. 1).
Table 1.
A list of studies demonstrating the probable mechanisms of ivermectin (IVM) against SARS-CoV-2
| Main role of ivermectin against SARS-CoV-2 | Study authors | Study year | References |
|---|---|---|---|
| A. Direct action on SARS-CoV-2 | |||
| Level 1: Action on SARS-CoV-2 cell entry | |||
| IVM docks in the region of leucine 91 of the spike protein and histidine 378 of the ACE-2 receptor | Leher et al. | 2020 | [21] |
| IVM has the highest binding affinity to the predicted active site of the S glycoprotein; Considerable binding affinity to the predicted active site of the SARS-CoV-2 RdRp protein; Highest binding affinity to the predicted active site of nsp14; highest binding affinity to the active site of the TMPRSS2 protein | Eweas et al. | 2021 | [22] |
| IVM utilizes viral spike protein, main protease, replicase, and human TMPRSS2 receptors as the most possible targets for executing its antiviral efficiency by disrupting binding | Choudhury et al. | 2021 | [23] |
| Level 2: Action on importin (IMP) superfamily | |||
| in presence of a viral infection, IVM targets the IMPα component of the IMP α/β1 heterodimer and binds to it, preventing interaction with IMP β1, subsequently blocking the nuclear transport of viral proteins | Yang et al. | 2020 | [25] |
| Level 3: Action as an ionophore | |||
| Two ivermectin molecules, reacting with each other in a “head-tail” mode, can create a complex suitable to be considered as an ionophore. These allow neutralizing the virus at an early stage of the infection before it adheres to the host cells and enters it | Rizzo | 2020 | [27] |
| Ivermectin acts as an ionophore by chloride channel upregulation to generate apoptosis and osmotic cell death | Dueñas-González et al.,Dominguez-Gomez et al. | 2021,2018 | [28],[29] |
| B. Action on host targets for viral replication | |||
| Level 4: Action as an antiviral | |||
| IVM has antiviral properties against other viruses including the RNA viruses such as Zika virus (ZKV), dengue virus, yellow fever virus (YFV), and West Nile virus (WNV), Hendra virus (HEV), Newcastle virus, Venezuelan equine encephalitis virus (VEEV), chikungunya virus (CHIKV), Semliki Forest virus (SFV), and Sindbis virus (SINV), Avian influenza A virus, porcine reproductive and respiratory syndrome virus (PRRSV), human immunodeficiency virus type 1 as well as DNA viruses such as equine herpesvirus type 1 (EHV-1) and pseudorabies virus (PRV) | Heidary et al. | 2020 | [30] |
| IVM acts as an inhibitor of HIV-1 nuclear protein transfer | Wagstaff et al. | 2011 | [31] |
| IVM causes a decrease in viral gene expression in BKPyV due to inhibition of nucleus entry | Bennett et al. | 2015 | [32] |
| IVM demonstrated IMP α/β-dependent nuclear transfer inhibition and reduced virus replication in a dose-dependent manner for BoHV-1 | Raza et al. | 2020 | [33] |
| Level 5: Action on viral replication and assembly | |||
| In Vero/hSLAM cells infected with the SARS-CoV-2 virus when “exposed” to 5 µM IVM showed a 5000-fold reduction in viral RNA at 48 h when compared to the control group | Caly et al. | 2020 | [34] |
| Utilizing modeling approach, predicted lung accumulation of ivermectin over ten times higher than EC 50 | Arshad et al. | 2020 | [35] |
| Best binding interaction between IVM and RNA-dependent RNA polymerase (RdRp) | Swargiary et al.a | 2020 | [37] |
| Highly efficient binding of IVM to nsp14 | Ma et al. | 2015 | [39] |
| Highly efficient binding of IVM to the viral N phosphoprotein and M protein | Eweas et al. | 2021 | [22] |
| Level 6: Action on posttranslational processing of viral polyproteins | |||
| IVM binds to both proteins, Mpro, and to a lesser extent to PLpro of SARS-CoV-2 | Eweas et al. | 2021 | [22] |
| IVM inhibits 3 chymotrypsin-like proteases | Mody et al. | 2021 | [40] |
| Level 7: Action on karyopherin (KPNA/KPNB) receptors | |||
| IVM inhibits the KPNA/KPNB1- mediated nuclear import of viral proteins | Caly et al. | 2020 | [34] |
| C. Action on host targets for inflammation | |||
| Level 8: Action on interferon (INF) levels | |||
| IVM promotes the expression of several IFN-related genes, such as IFIT1, IFIT2, IF144, ISG20, IRF9, and OASL | Seth et al. | 2016 | [45] |
| Level 9: Action on Toll- like receptors (TLRs) | |||
| IVM blocks activation of NF-kappa B pathway and inhibition of toll-like receptor 4 (TLR4) signaling | Zhang et al. | 2008 | [47] |
| Level 10: Action on nuclear factor-κB (NF-κB) pathway | |||
| IVM at its very low dose, which did not induce cytotoxicity, drastically reversed the resistance of tumor cells to the chemotherapeutic drugs both in vitro and in vivo by inhibition of the transcriptional factor NF-κB. | Jiang et al. | 2019 | [49] |
| IVM inhibits lipopolysaccharide (LPS)-induced production of inflammatory cytokines by blocking the NF-κB pathway and improving LPS-induced survival in mice | Zhang et al. | 2008 | [47] |
| Level 11: Action on the JAK-STAT pathway, PAI-1 and COVID-19 sequalae | |||
| IVM inhibits STAT-3, SARS-CoV-2-mediated inhibition of IFN and STAT 1, with the subsequent shift to a STAT-3- dominant signaling network that could result in almost all of the clinical features of COVID-19; STAT-3 acts as a “central hub” that mediates the detrimental COVID-19 cascade | Matsuyama et al. | 2020 | [44] |
| STAT-3 induces a C-reactive protein that upregulates PAI-1 levels. Ivermectin inhibits STAT-3 | Matsuyama et al. | 2020 | [44] |
| The PD-L1 receptors present on the endothelial cells are activated by STAT-3 causing T cell lymphopenia. IVM inhibits STAT-3 through direct inhibition | Matsuyama et al. | 2020 | [44] |
| Level 12: Action on P21 activated kinase 1 (PAK1) | |||
| IVM suppresses the Akt/mTOR signaling and promotes ubiquitin-mediated degradation of PAK1 hence compromising STAT-3 activity and decreasing IL-6 production | Dou et al. | 2016 | [59] |
| Level 13: Action on Interleukin-6 (IL-6) levels | |||
| IVM suppressed IL-6 and TNFα production | Zhang et al. | 2008 | [47] |
| IVM “dramatically reduced” IL-6/IL-10 ratio modulating infection outcomes | De Melo et al. | 2020 | [60] |
| Level 14: Action on allosteric modulation of P2X4 receptor | |||
| Positive allosteric modulation of P2X4 by IVM enhances ATP-mediated secretion of CXCL5 | Layhadi et al. | 2018 | [63] |
| Level 15: Action on high mobility group box 1 (HMGB1) | |||
| Ivermectin inhibits HMGB1 | Juarez et al. | 2018 | [65] |
| Level 16: Action as an immunomodulator on lung tissue and olfaction | |||
| No olfactory deficit was observed in IVM-treated females; IVM dramatically reduced the IL-6/IL-10 ratio in lung | De Melo et al. | 2020 | [60] |
| Level 17: Action as an anti-inflammatory | |||
| Anti-inflammatory action of IVM was explained as inhibition of cytokine production by lipopolysaccharide challenged macrophages, blockade of activation of NF-kB, and the stress-activated MAP kinases JNK and p38, and inhibition of TLR4 signaling | Zhang et al. | 2008 | [47] |
| Ci et al. | 2009 | [67] | |
| Yan et al. | 2011 | [68] | |
| Immune cell recruitment, cytokine production in bronchoalveolar lavage fluid, IgE, and IgG1 secretion in serum as well as hyper-secretion of mucus by goblet cells was reduced significantly by IVM | Yan et al. | 2011 | [68] |
| D. Action on other host targets | |||
| Level 18: Action on plasmin and annexin A2 | |||
| Annexin acts as a coreceptor for the conversion of plasminogen to plasmin in the presence of t-PA. increased levels of plasmin leads to direct activation of STAT-3 | Zaidi et al. | 2020 | [69] |
| IVM directly inhibits STAT-3 and could play a role in the inhibition of COVID-19 complications | Matsuyama et al. | 2020 | [44] |
| Level 19: Action on CD147 on the RBC | |||
| The SARS-CoV-2 does not internalize into the red blood cells but such attachments can lead to clumping. IVM binds to the S protein of the SARS-CoV-2 virus making it unavailable to bind with CD147 | Scheim et al. | 2020 | [70] |
| Level 20: Action on mitochondrial ATP under hypoxia on cardiac function | |||
| IVM increased mitochondrial ATP production by inducing Cox6a2 expression and maintains mitochondrial ATP under hypoxic conditions. This prevents pathological hypertrophy and improves cardiac function | Nagai et al. | 2017 | [72] |
aAvailable as preprint
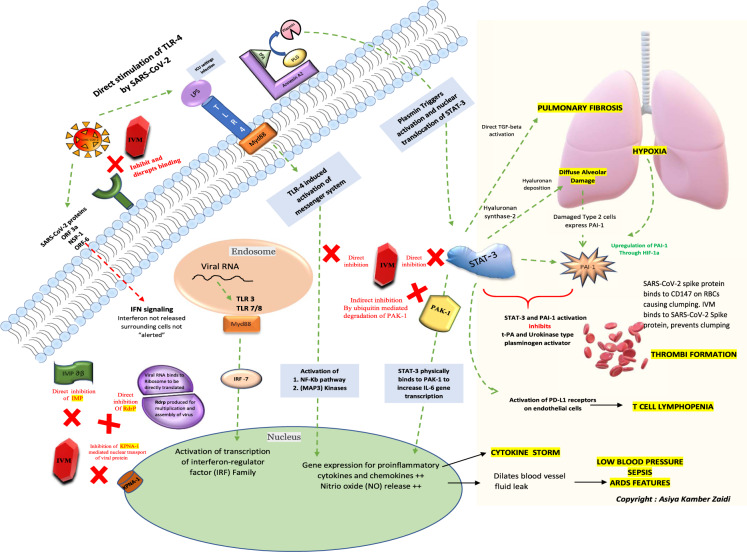
Methods
A comprehensive search of the PubMed database and available published literature was conducted from January 2008 up to September 2021 using syntax constructed using MeSH Database as follows: (stromectol OR Ivermectin OR “dihydroavermectin”) OR (22 AND 23-dihydroavermectin B) AND (antiviral OR virus OR COVID-19 OR SARS-CoV-2). The results obtained were manually reviewed for content, relevance and included when considered appropriate. The papers cited in the references were also reviewed and included when considered appropriate. The articles were retrieved manually to exclude any duplicates.
Results
Ivermectin as an antihelminth
Ivermectin has been approved as an antihelminthic [14]. It is a selective positive allosteric modulator at the glutamate-gated chloride channels found in nematodes and insects and acts by binding to these channels leading to chloride ion influx causing hyperpolarization of the cell and hence, dysfunction [15]. However, at higher concentrations, ivermectin can also bind to host GABA receptors only when the blood–brain barrier (BBB) is “leaky.” This is not the case in healthy human beings with an intact BBB as the drug is “excluded” by a P-glycoprotein drug pump (MDR-1). Chandler et al. considered ivermectin to be free of potential neurological adverse drug reactions, except in situations of overdose [16].
SARS-CoV-2 virus structure
SARS-CoV-2 is a sarbecovirus with structural similarity to SARS-CoV. Out of the four structural proteins of the SARS-CoV-2 beta coronavirus, namely: Spike (S) protein, membrane (M) protein, envelope (E) protein, and nucleocapsid (N) protein, the S protein is responsible for eliciting potent neutralizing antibody responses. The entry of SARS-CoV-2 into the host cell is mediated by the binding of the S1 subunit of its S protein (receptor binding domain) to the angiotensin-converting enzyme 2 (ACE-2) receptors present on the host cell surface [17]. The S2 subunit is associated with a fusion protein that binds with the cell membrane after priming with the transmembrane protease, serine 2 (TMPRSS2), and is responsible for fusion with the host cell.
The SARS-CoV-2 genome consists of ∼29.8 kb nucleotides; it possesses 14 open reading frames (ORFs) encoding 27 proteins [18]. The 5′ two-thirds of the viral genome encodes the replicase gene. It contains two ORFs: ORF1a and ORF1b. ORF1a/b encodes two polyproteins by polymerase frameshifting; these are then posttranslationally cleaved into 15 nonstructural proteins (nsps): nsp1–10 and nsp12–16. The rest of the genome encodes for the four structural proteins [(S protein, E protein, M protein, N protein], in addition to eight accessory proteins (3a/3b, p6, 7a/7b, 8b, 9b, and ORF14) [18]. The replicase also encodes the papain-like protease (PLpro) and the serine-type protease or main protease (Mpro) [19].
In principle, a molecule can act as an antiviral drug if it “inhibits some stage of the virus replication cycle, without being too toxic to the body’s cells.” [20]
The possible modes of action of antiviral agents would include the following:
- Inactivate extracellular virus particles.
- Prevent viral attachment and/or entry.
- Prevent replication of the viral genome.
- Prevent synthesis of specific viral protein(s).
- Prevent assembly or release of new infectious virions.
The role of ivermectin against the SARS-CoV-2 virus
The targets of activity of ivermectin can be divided into the following four groups:
A. Direct action on SARS-CoV-2
Level 1: Action on SARS-CoV-2 cell entry.
Level 2: Action on importin (IMP) superfamily.
Level 3: Action as an ionophore.
B. Action on host targets important for viral replication
Level 4: Action as an antiviral.
Level 5: Action on viral replication and assembly.
Level 6: Action on posttranslational processing of viral polyproteins.
Level 7: Action on karyopherin (KPNA/KPNB) receptors.
C. Action on host targets important for inflammation
Level 8: Action on interferon (INF) levels.
Level 9: Action on Toll-like receptors (TLRs).
Level 10: Action on nuclear factor-κB (NF-κB) pathway.
Level 11: Action on the JAK-STAT pathway, PAI-1, that could be involved with COVID-19 sequalae.
Level 12: Action on P21 activated kinase 1 (PAK1).
Level 13: Action on interleukin-6 (IL-6) levels.
Level 14: Action on allosteric modulation of P2X4 receptor.
Level 15: Action on high mobility group box 1 (HMGB1).
Level 16: Action as an immunomodulator on lung tissue and olfaction.
Level 17: Action as an anti-inflammatory.
D. Action on other host targets
Level 18: Action on plasmin and annexin A2.
Level 19: Action on CD 147 on the red blood cell (RBC).
Level 20: Action on mitochondrial ATP under hypoxia on cardiac function.
The direct “antiviral targets” may be useful in the early stages while the anti-inflammatory targets might be addressed in the later stages of the disease.
Direct action of ivermectin on SARS-CoV-2
Level 1: Action on SARS-CoV-2 cell entry
A study by Lehrer et al. observed that ivermectin docked in the region of leucine 91 of the SARS-CoV-2 spike protein and histidine 378 of the host cell ACE-2 receptor blocking its entry into the host cell [21]. In yet another study by Eweas et al., potential repurposed drugs such as ivermectin, chloroquine, hydroxychloroquine, remdesivir, and favipiravir were screened and molecular docking with different SARS-CoV-2 target proteins including S and M proteins, RNA-dependent RNA polymerase (RdRp), nucleoproteins, viral proteases, and nsp14, was performed. Ivermectin showed the following 5 important docking properties [22]:
- Highest binding affinity to the predicted active site of the S glycoprotein (Moldock score −140.584) and protein–ligand interactions (Moldock score −139.371).
- Considerable binding affinity to the predicted active site of the SARS-CoV-2 RdRp protein (Moldock score −149.9900) and protein–ligand interactions (Moldock score −147.608), it formed H-bonds with only two amino acids: Cys622 and Asp760.
- Highest binding affinity (Moldock score −212.265) to the predicted active site of nsp14.
- The highest binding affinity to the active site of the TMPRSS2 protein (Moldock score −174.971) and protein–ligand interactions (Moldock score −180.548). Moreover, it formed five H-bonds with Cys297, Glu299, Gln438, Gly462, and Gly464 amino acid residues present at the predicted active site of the TMPRSS protein.
- The free binding energy of the spike protein (open) was higher in ivermectin (−398.536 kJ/mol) than remdesivir (−232.973 kJ/mol).
An in silico data analysis conducted by Choudhury et al. demonstrated that ivermectin efficiently utilizes viral spike protein, main protease, replicase, and human TMPRSS2 receptors as the most possible targets for executing its “antiviral efficiency” by disrupting binding. Since ivermectin exploits protein targets from both, the virus and humans, this could be responsible for its excellent in vitro activity against SARS-CoV-2 [23].
The development of vaccines for SARS-CoV-2 is centered around spike protein biology (virus targeted) and the recently documented “vaccine escape strains” have been a cause of worry. In such a situation, ivermectin is both, virus as well as host targeted and hence could act as a potential therapeutic against these new strains that could “escape” immunity offered by the vaccine.
Level 2: Action on IMP superfamily
Inside the cell, the nuclear transport of proteins into and out of the nucleus is signal-dependent and mediated by the IMP superfamily of proteins that exist in α and β forms. This IMPα/β1 exists as a heterodimer with a “IBB” (IMP β-binding) site present over IMP α that binds to IMP β1 on “cargo recognition” by IMPα. The SARS-CoV-2 virus upon host cell entry tends to “load” its proteins over the host protein IMP α/β1 heterodimer (IMP) to enter the nucleus through the nuclear pore complex. Once inside, the IMP molecule detaches while the viral protein from the SARS-CoV-2 virus hijacks the host cell machinery and inhibits the natural cell “antiviral” response by blocking the release of INF (an antiviral substance released by an infected cell to alert the surrounding cells of an ongoing viral attack). As a result, the surrounding cells become “unsuspecting victims” of the virus and the infection continues with the virus escaping recognition by the immune cells [24]. Ivermectin, in presence of a viral infection, targets the IMPα component of the IMP α/β1 heterodimer and binds to it, preventing interaction with IMP β1, subsequently blocking the nuclear transport of viral proteins. This allows the cell to carry out its normal antiviral response [25]. In such a case, it should be noted that the activity of ivermectin here is virostatic, that is, it neutralizes the virus by competing for the same receptor.
Level 3: Action as an ionophore
Ionophores are molecules that typically have a hydrophilic pocket which constitutes a specific binding site for one or more ions (usually cations), while its external surface is hydrophobic, allowing the complex thus formed to cross the cell membranes, affecting the hydro-electrolyte balance [26]. It can be hypothesized that two ivermectin molecules, reacting with each other in a “head-tail” mode, can create a complex suitable to be considered such [27]. These ionophores allow neutralizing the virus at an early stage of the infection before it can adhere to the host cells and enter it to exploit their biochemical machinery for the production of other viral particles. Ivermectin acts as an ionophore by chloride channel upregulation to generate apoptosis and osmotic cell death [28, 29].
Action on host targets for viral replication
Level 4: Action as an antiviral
A systematic review article by Heidary et al. discussed the “antiviral” properties of ivermectin against other viruses including the RNA viruses such as Zika virus, dengue virus, yellow fever virus, and West Nile virus, Hendra virus, Newcastle virus, Venezuelan equine encephalitis virus (VEEV), chikungunya virus, Semliki Forest virus, and Sindbis virus, Avian influenza A virus, porcine reproductive and respiratory syndrome virus, human immunodeficiency virus type 1 as well as DNA viruses such as equine herpesvirus type 1, BK polyoma virus (BKPyV), porcine circovirus 2 (PCV2), bovine herpesvirus 1 virus (BoHV-1) and pseudorabies virus [30]. Ivermectin inhibited proliferation of DENV by blocking nonstructural protein 5 interaction with IMP α/β transporter, reduced nuclear-associated capsid, virus titer, and cytopathic effects caused by the VEEV in addition to reduction in viral replication, nuclear accumulation of capsid protein in the VEEV infected cells [30]. In an in vitro study by Wagstaff et al., the researchers evaluated the effects of ivermectin as an inhibitor of HIV-1 nuclear protein transfer. The study concluded that ivermectin is a nuclear transport inhibitor via IMPα/β, but does not affect the nuclear transfer via IMPβ1 alone, and completely inhibits nuclear import of the active integrase protein of HIV-1 as a critical component of the preintegration complex [31]. Moreover, ivermectin led to a decrease in viral gene expression due to inhibition of nucleus entry, indicating that (BKPyV), has access to the nucleus through active nuclear pore complex transfer [32]. Ivermectin demonstrated IMP α/β-dependent nuclear transfer inhibition and reduced virus replication in a dose-dependent manner for BoHV-1 and nuclear localization signal (NLS)-mediated nuclear import pathway for PCV2 [33].
Level 5: Action on viral replication and assembly
An in vitro study by Caly et al. demonstrated that Vero/hSLAM cells infected with the SARS-CoV-2 virus when “exposed” to 5 µM ivermectin showed a 5000-fold reduction in viral RNA at 48 h when compared to the control group [34]. This study attracted opinions regarding the inability of ivermectin to achieve the therapeutic effect of COVID-19 through routine dosage. Contrary to this, Arshad et al., by utilizing the modeling approach, predicted lung accumulation of ivermectin over ten times higher than EC50. This likelihood of attainment of higher lung tissue concentrations of ivermectin leaves the door open for further research, especially for respiratory infections [35].
An explanation for the study by Caly et al. was provided in a review article: Global trends in clinical studies of ivermectin in COVID-19 by Yagisawa et al., coauthored by Prof. Satoshi Omura, regarding the “setting of the sensitivity for experimental systems in vitro.” As per the authors, using Vero/hSLAM cells, the antiviral activity of the test drug was reliably measured and the sensitivity of the IC50 = 2 μM set by them was appropriate as neither false positives nor false negatives occurred. Therefore, the study by Caly et al. merely indicated that ivermectin was found to have anti-SARS-CoV-2 activity in vitro—no more, no less. Also, the fact that there are in vivo infection experiments that could be used to connect in vitro experiments to clinical studies [36].
Another in silico study by Swargiary et al. demonstrated the best binding interaction of −9.7 kcal/mol between ivermectin and RdRp suggesting inhibition of viral replication [37]. The RdRP residing in nsp12 is the centerpiece of the coronavirus replication and transcription complex and has been suggested as a promising drug target as it is a crucial enzyme in the virus life cycle both for replication of the viral genome but also for transcription of subgenomic mRNAs [38]. Ivermectin binds to the viral Rdrp and disrupts it. The highly efficient binding of ivermectin to nsp14 confirms its role in inhibiting viral replication and assembly. It is well known that nsp14 is essential in transcription and replication. It acts as a proofreading exoribonuclease and plays a role in viral RNA capping by its methyltransferase activity [39]. Moreover, highly efficient binding of ivermectin to the viral N phosphoprotein and M protein is suggestive of its role in inhibiting viral replication and assembly [22].
Level 6: Action on posttranslational processing of viral polyproteins
Once gaining entry into the host cell, the viral RNA is translated by the host ribosome into a large “polyprotein.” Some enzymes break away through autoproteolysis from this polyprotein and further help other proteins to break off and carry out their function for replication. One such enzyme, 3 chymotrypsin-like proteases (3′cl pro/Mpro) is responsible for working on this polyprotein causing other proteins to “librate” and carry out viral replication. Ivermectin binds to this enzyme and disrupts it [40]. It also efficiently binds to both proteins, Mpro, and to a lesser extent to PLpro of SARS-CoV-2; therefore, it has a role in preventing the posttranslational processing of viral polyproteins [22].
Level 7: Action on karyopherin (KPNA/KPNB) receptors
Karyopherin-α1 (KPNA1) is essential for the nuclear transport of signal transducers and activators of transcription 1 (STAT1) [41], and the interaction between STAT1 and KPNA1 (STAT1/KPNA1) involves a nonclassical NLS. Ivermectin inhibits the KPNA/KPNB1- mediated nuclear import of viral proteins allowing the cell to carry out its normal antiviral response [34].
Action on host targets for inflammation
Level 8: Action on INF levels
Virus-infected cells release INFs that bind to the IFN receptors present on neighboring cells alerting them of a viral attack. The IFN-I and IFN-III receptors then further activate members of the JAK-STAT family. The virus after gaining entry into the host cell hijacks the host cell machinery and works towards antagonizing the normal INF-mediated host cell antiviral response. SARS-CoV-2 proteins such as ORF3a, NSP1, and ORF6 inhibit IFN-I signaling [42, 43]. As a result, the cells surrounding the SARS-CoV-2 virus-infected cell “fail” to receive “critical and protective IFN signals” causing this SARS-CoV-2 virus to replicate and spread without any hindrance. This is one of the main reasons that, at this stage, COVID-19 infection is “hard to detect” clinically [44].
Ivermectin has been shown to promote the expression of several IFN-related genes, such as IFIT1, IFIT2, IF144, ISG20, IRF9, and OASL [45].
Level 9: Action on TLRs
Upon virus entry, the intracellular pattern recognition receptors (PRRs) present on the host cells are responsible for detecting the viral attack. The virus activates one such PRR named the TLRs. These receptors are present on various immune system cells that help them locate and bind with the pathogen. The activation of TLRs, causes oligomerization, further activating downstream INF regulatory factors (IRFs) and NF-kB transcription factors inducing INF production [46]. Ivermectin plays a role in the blockade of activation of NF-kB pathway and inhibition of TLR4 signaling [47].
Level 10: Action on NF-κB pathway
Activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway induces the expression of various proinflammatory genes, including those encoding cytokines and chemokines [48]. Jiang et al. demonstrated that ivermectin at its very low dose, which did not induce cytotoxicity, drastically reversed the resistance of tumor cells to the chemotherapeutic drugs both in vitro and in vivo by inhibition of the transcriptional factor NF-κB [49]. Also, Zhang et al. suggested that ivermectin inhibits lipopolysaccharide (LPS)-induced production of inflammatory cytokines by blocking the NF-κB pathway and improving LPS-induced survival in mice [47]. Therefore, using ivermectin could be helpful in ICU settings where there are increased chances of bacterial infections (LPS mediated).
Level 11: Action on the JAK-STAT pathway, PAI-1 and COVID-19 sequalae
A strong correlation exists between SARS-CoV-2 viral load, disease severity, and progression [50]. COVID-19 not only causes flu-like symptoms such as fever, dry cough but could also lead to widespread thrombosis with microangiopathy in pulmonary vessels [51], raise D-dimer levels [52], cause lymphopenia [53], raise proinflammatory cytokine and chemokine production [54] as well as lead to a significant elevation of CRP levels [55] SARS-CoV-2 has structural similarity with SARS-CoV. Several SARS-CoV proteins antagonize the antiviral activities of IFNs and the downstream JAK (Janus kinase)-STAT signaling pathways they activate. JAK family kinases display a wide range of functions in ontogeny, immunity, chronic inflammation, fibrosis, and cancer [56].
The host proteins, such as the members of the STATs and NF-κB, enter the nucleus through nuclear envelope-embedded nuclear pores mediated by the IMPα/β1 heterodimer and play a role in COVID-19 pathogenesis. Frieman et al. demonstrated that accessory SARS ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane [57]. A review article by Matsuyama et al. hinted at SARS-CoV-2-mediated inhibition of IFN and STAT 1, with the subsequent shift to a STAT-3 dominant signaling network that could result in almost all of the clinical features of COVID-19 [44].
Before discussing further, it is important to understand the link between STAT-3 upregulation and COVID-19 sequelae and the role of ivermectin in inhibiting STAT-3. STAT-3 acts as a “central hub” that mediates the detrimental COVID-19 cascade. In the lungs, STAT-3 activates hyaluronan synthase 2 leading to deposition of hyaluronan causing diffuse alveolar damage. The damaged type 2 alveolar cells express PAI-1 (plasminogen activator inhibitor-1). In addition, hypoxia due to diffuse alveolar damage causes an upregulation of PAI-1 through HIF-1a. STAT-3 also directly activates PAI-1. The simultaneous activation of PAI-1 and STAT-3 inhibits t-PA and urokinase-type plasminogen activator leading to thrombi formation in the capillaries. PAI-1 also binds to TLR4 receptors on macrophages further activating the NF-kB pathway.
The “cytokine storm” typical of severe COVID-19 involves STAT-3 mediated upregulation of proinflammatory cytokines, TNFα, and IL-6 in macrophages. In addition, STAT-3 induces a C-reactive protein that upregulates PAI-1 levels. STAT-3 is directly responsible for activating IL-6 gene transcription which further leads to an increase in TGF-β causing pulmonary fibrosis. The PD-L1 receptors present on the endothelial cells are activated by STAT-3 causing T cell lymphopenia. Ivermectin inhibits STAT-3 through direct inhibition that could be helpful in decreasing COVID-19 sequelae [44].
Level 12: Action on PAK1
The PAK1 physically binds to both JAK1 and STAT-3, and the resultant PAK1/STAT-3 complex activates IL-6 gene transcription responsible for cytokine storm in COVID-19 [58]. Ivermectin suppresses the Akt/mTOR signaling and promotes ubiquitin-mediated degradation of PAK1 hence compromising STAT-3 activity and decreasing IL-6 production [59].
Level 13: Action on IL-6 levels
A study by Zhang et al. demonstrated that ivermectin suppressed IL-6 and TNFα production, two major components of the detrimental cytokine storm induced by SARS-CoV-2 and “dramatically reduced” IL-6/IL-10 ratio modulating infection outcomes [47, 60].
Level 14: Action on allosteric modulation of P2X4 receptor
P2X receptors are the channels selective to cation, are gated by extracellular ATP [61] and mediate several functions in health and disease [62]. From the seven subunits of P2X receptors, P2X4 is most sensitive to ivermectin. Positive allosteric modulation of P2X4 by ivermectin enhances ATP-mediated secretion of CXCL5 (proinflammatory chemokine). CXCL5 is a chemo-attractant molecule expressed in inflammatory cells in different tissues and modulates neutrophil chemotaxis and chemokine scavenging [63].
Level 15: Action on HMGB1
The damage-associated molecular pattern HMGB1 is released by damaged cells acting as an agonist for the TLR4 receptor and hence mediating lung inflammation associated with COVID-19 [64]. Ivermectin inhibits HMGB1 [65].
Level 16: Action as an immunomodulator on lung tissue and olfaction
In a study by De Melo et al., the effects of ivermectin were investigated on SARS-CoV-2 infection using the golden Syrian hamster as a model for COVID-19. Both male and female adult golden Syrian hamsters were intranasally inoculated with 6 × 104 PFU of SARS-CoV-2. At the time of infection, animals received a single subcutaneous injection of ivermectin (antiparasitic dose of 400 μg/kg) classically used in a clinical setting and were monitored over 4 days. Mock-infected animals received the physiological solution only. Interestingly, ivermectin had a sex-dependent and compartmentalized immunomodulatory effect, preventing clinical deterioration and reducing the olfactory deficit in infected animals. This effect was sex-dependent: infected males presented a reduction in the clinical score whereas a complete absence of signs was noticed in the infected females. Regarding the olfactory performance, 83.3% (10/12) of the saline-treated males presented with hyposmia/anosmia, in contrast to only 33.3% (4/12) of ivermectin treated males (Fisher’s exact test p = 0.036). No olfactory deficit was observed in ivermectin treated females (0/6), while 33.3% (2/6) of saline-treated females presented with hyposmia/anosmia (Fisher’s exact test p = 0.455). Ivermectin dramatically reduced the IL-6/IL-10 ratio in lung tissue, which likely accounts for the more favorable clinical presentation in treated animals [60]. Loss of smell has been reported as one of the common symptoms in COVID-19 [66]. Interestingly, majority of patients in India regained their sense of smell after a brief anosmic period during their clinical course. It could be hypothesized that ivermectin might have a role to play in reducing SARS-CoV-2 induced olfactory deficit.
Level 17: Action as an anti-inflammatory
The mechanism for anti-inflammatory action of ivermectin was explained as inhibition of cytokine production by LPS challenged macrophages, blockade of activation of NF-kB, and the stress-activated MAP kinases JNK and p38, and inhibition of TLR4 signaling [47, 67]. Moreover, Immune cell recruitment, cytokine production in bronchoalveolar lavage fluid, IgE, and IgG1 secretion in serum as well as hyper-secretion of mucus by goblet cells was reduced significantly by ivermectin [68].
Action on other host targets
Level 18: Action on plasmin and annexin A2
As per the study by Zaidi et al., annexin A2 may be linked to COVID-19 pathophysiology [69]. Annexin A2 acts as a coreceptor for the conversion of plasminogen to plasmin in the presence of t-PA. Increased plasmin levels are found in comorbid states and are also responsible for the early stages of viral infection. Plasmin leads to direct activation of STAT-3 inducing detrimental COVID-19 sequelae. Ivermectin directly inhibits STAT-3 and could play a role in the inhibition of COVID-19 complications.
Level 19: Action on CD147 on the RBC
The transmembrane receptor CD147, present on the RBC along with ACE-2 has been recognized as a key binding site for SARS-CoV-2 spike protein. The SARS-CoV-2 does not internalize into the RBC but such attachments can lead to clumping. Ivermectin binds to the S protein of the virus making it unavailable to bind with CD147 [70]. This action might also be beneficial in advanced stages of COVID-19 presenting with clotting/thrombotic phenomena.
Level 20: Action on mitochondrial ATP under hypoxia on cardiac function
SARS-CoV-2 has been a well-known cause for acute myocardial injury and chronic damage to the cardiovascular system in active infection as well as in long haulers [71]. Nagai et al. demonstrated that ivermectin increased mitochondrial ATP production by inducing Cox6a2 expression and maintains mitochondrial ATP under hypoxic conditions preventing pathological hypertrophy and improving cardiac function [72].
Conclusion
We have summarized published results on the inhibition of multiple viral and host targets that could be involved in SARS-CoV-2 replication and the disease COVID-19. Although multiple antiviral and host target activities have been reported for ivermectin in SARS-CoV-2 and COVID-19, it is still unclear if any of these activities will play a role in the prevention and treatment of the disease. The controlled clinical trials that are underway will reveal if these activities will translate into clinical efficacy.
Acknowledgements
The authors would like to thank all the academicians, physicians and scientists dedicating their efforts and time to COVID-19 research. The authors would like to specially thank children who bring positivity and hope in these difficult times, especially Ginevra Dehgani.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
This is a revised version of the previously retracted article below. The article has been revised to remove references to clinical studies, focusing exclusively on the mechanisms of action of ivermectin. The article has undergone peer review independent of the original article’s review process.
Zaidi, A.K., Dehgani-Mobaraki, P. RETRACTED ARTICLE: The mechanisms of action of Ivermectin against SARS-CoV-2: An evidence-based clinical review article. J Antibiot (2021). 10.1038/s41429-021-00430-5.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gharebaghi R, Heidary F. COVID-19 and Iran: swimming with hands tied! Swiss Med Wkly. 2020;150:w20242. doi: 10.4414/smw.2020.20242.. [DOI] [PubMed] [Google Scholar]
- 2.Crump A, Ōmura S. Ivermectin, ‘wonder drug’ from Japan: the human use perspective. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:13–28. doi: 10.2183/pjab.87.13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kircik LH, Del Rosso JQ, Layton AM, Schauber J. Over 25 years of clinical experience with ivermectin: an overview of safety for an increasing number of indications. J Drugs Dermatol. 2016;15:325–32. [PubMed] [Google Scholar]
- 4.Gonzalez Canga A, et al. The pharmacokinetics and interactions of ivermectin in humans–a mini-review. AAPS J. 2008;10:42–6. doi: 10.1208/s12248-007-9000-9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar BS, Jeyaraman M, Jain R, Anudeep TC. A wonder drug in the arsenal against COVID—19: medication evidence from ivermectin. J Adv Med Med Res. 2020;32:30–7. doi: 10.9734/jammr/2020/v32i1030512. [DOI] [Google Scholar]
- 6.Novac N. Challenges and opportunities of drug repositioning. Trends Pharm Sci. 2013;34:267–72. doi: 10.1016/j.tips.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 7.ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2021 [cited 10 November 2021]. Available from: https://clinicaltrials.gov/ct2/home Home – ClinicalTrials.gov.
- 8.Activ6study.org. Activ-6 study. Activ6study.org; 2021. https://activ6study.org/.
- 9.Principletrial.org. Join the PRINCIPLE Trial. Principletrial.org.; 2021. https://www.principletrial.org/.
- 10.Edwards G, Dingsdale A, Helsby N, Orme ML, Breckenridge AM. The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution. Eur J Clin Pharm. 1988;35:681–4. doi: 10.1007/BF00637608. [DOI] [PubMed] [Google Scholar]
- 11.Verrest L, Dorlo TPC. Lack of clinical pharmacokinetic studies to optimize the treatment of neglected tropical diseases: a systematic review. Clin Pharmacokinet. 2017;56:583–606. doi: 10.1007/s40262-016-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klotz U, Ogbuokiri JE, Okonkwo PO. Ivermectin binds avidly to plasma proteins. Eur J Clin Pharmacol. 1990;39:607–8. doi: 10.1007/BF00316107.. [DOI] [PubMed] [Google Scholar]
- 13.Wu MA, Fossali T, et al. Hypoalbuminemia in COVID-19: assessing the hypothesis for underlying pulmonary capillary leakage. J Intern Med. 2021. 10.1111/joim.13208. [DOI] [PubMed]
- 14.World Health Organization. Application for inclusion of ivermectin on the WHO Model List of Essential Medicines (EML) and Model List of Essential Medicines for Children (EMLc). World Health Organization; 2016.
- 15.Martin RJ, Robertson AP, Choudhary S. Ivermectin: an anthelmintic, an insecticide, and much more. Trends Parasitol. 2021;37:48–64. doi: 10.1016/j.pt.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler RE. Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg. 2018;98:382–8. doi: 10.4269/ajtmh.17-0042.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.03.045.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020. 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed]
- 19.Mielech AM, Kilianski A, Baez-Santos YM, Mesecar AD, Baker SC. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology. 2014;450–451:64–70. doi: 10.1016/j.virol.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehigh.edu. BioS 353. Lehigh.edu; 2021. https://www.lehigh.edu/~jas0/V14.html.
- 21.Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo. 2020;34:3023–6. doi: 10.21873/invivo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eweas AF, Alhossary AA, Abdel-Moneim AS. Molecular docking reveals ivermectin and remdesivir as potential repurposed drugs against SARS-CoV-2. Front Microbiol. 2021;11:592908.. doi: 10.3389/fmicb.2020.592908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhury A, Das NC, Patra R, Bhattacharya M, Ghosh P, Patra BC, et al. Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach. Future Virol. 2021. 10.2217/fvl-2020-0342.
- 24.Fulcher A, Jans DA. Regulation of nucleocytoplasmic trafficking of viral proteins; an integral role in pathogenesis? Biochem Biophys Acta Mol Cell Res. 2011;1813:2176–90. doi: 10.1016/j.bbamcr.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang SNY, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir Res. 2020;177:104760. doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- 26.Freedman JC. Chapter 4—Ionophores in planar lipid bilayers. In: Sperelakis N, editor. Cell physiology sourcebook. Essentials of membrane biophysics, 4th ed. London, UK; Waltham, MA, USA; San Diego, CA, USA: Academic Press; 2012. p. 61–6.
- 27.Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch Pharm. 2020;393:1153–6. doi: 10.1007/s00210-020-01902-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dueñas-González A, Juárez-Rodríguez M. Ivermectin: potential repurposing of a versatile antiparasitic as a novel anticancer. In: Raymond C, Dalgleish A, editors. Repurposed drugs for cancer. IntechOpen; 2021. 10.5772/intechopen.99813.
- 29.Dominguez‑Gomez G, Chavez‑Blanco A, Medina‑Franco JL, Saldivar‑Gonzalez F, Flores‑Torrontegui Y, Juarez M, et al. Ivermectin as an inhibitor of cancer stem‑like cells. Mol Med Rep. 2018;17:3397–403. doi: 10.3892/mmr.2017.8231.. [DOI] [PubMed] [Google Scholar]
- 30.Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020;73:593–602. doi: 10.1038/s41429-020-0336-z.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagstaff KM, Rawlinson SM, Hearps AC, Jans DA. An AlphaScreen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import. J Biomol Screen. 2011;16:192–200. doi: 10.1177/1087057110390360.. [DOI] [PubMed] [Google Scholar]
- 32.Bennett SM, Zhao L, Bosard C, Imperiale MJ. Role of a nuclear localization signal on the minor capsid proteins VP2 and VP3 in BKPyV nuclear entry. Virology. 2015;474:110–6. doi: 10.1016/j.virol.2014.10.013.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raza S, Shahin F, Zhai W, et al. Ivermectin inhibits bovine herpesvirus 1 DNA polymerase nuclear import and interferes with viral replication. Microorganisms. 2020;8. 10.3390/microorganisms8030409. [DOI] [PMC free article] [PubMed]
- 34.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;104787. 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed]
- 35.Arshad U, Pertinez H, Box H, et al. Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin Pharmacol Ther. 2020. 10.1002/cpt.1909. [DOI] [PMC free article] [PubMed]
- 36.Yagisawa M, Foster PJ, Hanaki H, Ōmura S. Global trends in clinical studies of ivermectin in COVID-19. Jpn J Antibiotics. 2021;44:74-1.
- 37.Swargiary A. Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: evidence from in silico studies. Research Square; 2020. 10.21203/rs.3.rs-73308/v1.
- 38.V’kovski P, Kratzel A, Steiner S, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–70. doi: 10.1038/s41579-020-00468-6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Wu L, Shaw N, Gao Y, Wang J, Sun Y, et al. Structural basis and functional analysis of the SARS coronavirus nsp14-nsp10 complex. Proc Natl Acad Sci USA. 2015;112:9436–41. doi: 10.1073/pnas.1508686112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mody V, Ho J, Wills S, Mawri A, Lawson L, Ebert MCCJC, et al. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun Biol. 2021;4:93. doi: 10.1038/s42003-020-01577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 1997;16:7067–77. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konno Y, Kimura I, Uriu K, Fukushi M, Irie T, Koyanagi Y, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant. Cell Rep. 2020. 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed]
- 43.Yang D, Chu H, Hou Y, Chai Y, Shuai H, Lee AC-Y, et al. Attenuated interferon and pro-inflammatory response in SARSCoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J Infect Dis. 2020. 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed]
- 44.Matsuyama T, Kubli SP, Yoshinaga SK, et al. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020;27:3209–25. doi: 10.1038/s41418-020-00633-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seth C, Mas C, Conod A, Mueller J, Siems K, Kuciak M, et al. LongLasting WNT-TCF response blocking and epigenetic modifying activities of withanolide f in human cancer cells. PLoS ONE. 2016;11:e0168170. doi: 10.1371/journal.pone.0168170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park A, Iwasaki A, Type I. and type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–8. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Song Y, Ci X, et al. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res. 2008;57:524–9. doi: 10.1007/s00011-008-8007-8. [DOI] [PubMed] [Google Scholar]
- 48.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, Wang P, Sun YJ, Wu YJ. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway. J Exp Clin Cancer Res. 2019;38:265. doi: 10.1186/s13046-019-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARSCoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed]
- 52.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020. 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed]
- 53.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19:18. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bharadwaj U, Kasembeli MM, Robinson P, Tweardy DJ. Targeting janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Pharm Rev. 2020;72:486–526. doi: 10.1124/pr.119.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/golgi membrane. J Virol. 2007;81:9812–24. doi: 10.1128/JVI.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J-H, Choi HS, Kim S-L, Lee D-S. The PAK1-Stat3 signaling pathway activates IL-6 gene transcription and human breast cancer stem cell formation. Cancers. 2019;11:1527. doi: 10.3390/cancers11101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dou Q, Chen H-N, Wang K, Yuan K, Lei Y, Li K, et al. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. 2016;76:4457–69. doi: 10.1158/0008-5472.CAN-15-2887. [DOI] [PubMed] [Google Scholar]
- 60.De Melo GD, Lazarini F, Larrous F, et al. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol Med. 2021;13:e14122. doi: 10.15252/emmm.202114122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–93. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stokes L, Layhadi JA, Bibic L, Dhuna K, Fountain SJ. P2X4 receptor function in the nervous system and current breakthroughs in pharmacology. Front Pharm. 2017;8:291. doi: 10.3389/fphar.2017.00291.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Layhadi JA, Turner J, Crossman D, Fountain SJ. ATP evokes Ca2+ responses and CXCL5 secretion via P2X4 receptor activation in human monocyte-derived macrophages. J Immunol. 2018;200:1159. doi: 10.4049/jimmunol.1700965.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersson U, Ottestad W, Tracey KJ. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 2020;26:42. 10.1186/s10020-020-00172-4pmid. [DOI] [PMC free article] [PubMed]
- 65.Juarez M, Schcolnik-Cabrera A, Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am J Cancer Res. 2018;8:317–31. [PMC free article] [PubMed] [Google Scholar]
- 66.Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, et al. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020;20:1015–6. doi: 10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ci X, Li H, Yu Q. Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam Clin Pharm. 2009;23:449–55. doi: 10.1111/j.1472-8206.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- 68.Yan S, Ci X, Chen N. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm Res. 2011;60:589–96. doi: 10.1007/s00011-011-0307-8. [DOI] [PubMed] [Google Scholar]
- 69.Zaidi AK, Dawoodi S, Pirro M, Monti M, Mobaraki PD. Key role of annexin A2 and plasmin in COVID-19 pathophysiology, clinical presentation and outcomes—a review. Ital J Prev, Diagn Ther Med. 2020;3. 10.30459/2020-24.
- 70.Scheim DE. Ivermectin for COVID-19 Treatment: Clinical Response at Quasi-Threshold Doses Via Hypothesized Alleviation of CD147-Mediated Vascular Occlusion (June 26, 2020). Available at SSRN: https://ssrn.com/abstract=3636557.
- 71.Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60. doi: 10.1038/s41569-020-0360-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagai H, Satomi T, Abiru A, Miyamoto K, Nagasawa K, Maruyama M, et al. Antihypertrophic effects of small molecules that maintain mitochondrial ATP levels under hypoxia. EBioMedicine. 2017;24:147–58. doi: 10.1016/j.ebiom.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Articles from The Journal of Antibiotics are provided here courtesy of Nature Publishing Group

