Cellular Therapy for Knees | Patient Success Story: How Stem Cell Therapy Improved Rusty’s Knees
Cellular Therapy provides promising results in treating osteoarthritis of the knees. In this testimonial, Rusty shares the results of his stem cell therapy and how it improved the function of his knee. Four months later, he reports a great improvement in function and less pain using cellular therapy.
The Hansen Clinic of Natural Medicine uses the highest grade allogeneic stem cells donated from umbilical cord blood of healthy mothers. Our cellular therapy partner Bio-Genix ensures that all donors pass a rigorous screening and that the stem cells optimal for use in regenerative therapy applications.
Dr. Hansen’s Recommendation for Cellular Therapy
Cellular Therapy is an effective treatment for damaged knees. Patients experiencing knee pain now have alternatives to invasive surgery that requires hospitalization or an out patient procedure and comes with certain risks and a prolonged recovery time. Cellular Therapy provides effective regenerative treatment in addressing the effects of osteoarthritis and injury due to trauma. Stem Cell Therapy is a safe and effective option for patients seeking pain relief and improved mobility without the side effects of a surgical procedure.
Interested in learning more about Stem Cell Therapy for your knees?
Stem cell application for osteoarthritis in the knee joint: A minireview
World J Stem Cells. 2014 Nov 26; 6(5): 629–636.
Published online 2014 Nov 26. doi: 10.4252/wjsc.v6.i5.629
Kristin Uth and Dimitar Trifonov
Abstract
Knee osteoarthritis is a chronic, indolent disease that will affect an ever increasing number of patients, especially the elderly and the obese. It is characterized by degeneration of the cartilage substance inside the knee which leads to pain, stiffness and tenderness. By some estimations in 2030, only in the United States, this medical condition will burden 67 million people. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, restoration of normal cartilage function has been difficult to achieve. Moreover, in severe cases of knee osteoarthritis total knee replacement may be required. Total knee replacements come together with high effort and costs and are not always successful. The aim of this review is to outline the latest advances in cellular therapy for knee osteoarthritis as well as highlight some of the advantages of cellular therapy over traditional approaches aimed at restoration of cartilage function in the knee. In addition to the latest advances in the field, challenges associated with cellular therapy regarding knee cartilage regeneration and chondrogenesis in vitro and in vivo are also outlined and analyzed. Furthermore, based on their critical assessment of the present academic literature the authors of this review share their vision about the future of stem cell applications in the treatment of knee osteoarthritis.
Keywords: Multipotent adult mesenchymal stem cells, Osteoarthritis, Knee joint, Clinical trial
Core tip: Knee osteoarthritis is a common medical condition in the elderly and the obese. Despite the variety of available conventional treatments for this disease, in recent years cellular therapy has been applied in an ever increasing number of clinical cases. Therefore the aim of this review is to outline the latest advances in cellular therapy as a non-pharmacologic treatment for knee osteoarthritis. It also emphasizes on some of the challenges associated with cellular therapy regarding knee cartilage regeneration and chondrogenesis in vitro and in vivo.
Introduction
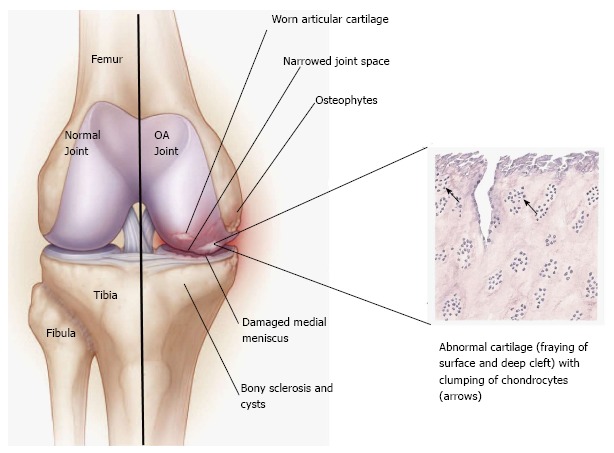
Osteoarthritis (OA) of the knee is a chronic, indolent disease that affects all genders, ages and races but is known to be most common in the elderly and in obese people. A degenerative disease of the connective tissue, it mainly affects the articular cartilage (Figure (Figure11)[1]. The definition of knee OA varies in reported studies and includes self-reported knee OA (obtained from a questionnaire), radiographic definitions of knee osteoarthritis, and symptomatic knee OA (self-reported joint pain and radiographic evidence of OA)[2]. Symptoms may include joint pain, stiffness and tenderness. Furthermore, as the cartilage substance decreases, the bone surface may also become affected. This results in development of osteophytes (bone spurs) and direct bone-bone contact. In addition to the stiffness of the joint, the patient tries to avoid pain by minimizing joint movement, which leads to muscle atrophy and laxity of the ligaments[1-4].
Figure 1 (See above)
Pathophysiology of knee osteoarthritis. Comparison between a normal and diseased joint (Illustration created after Felson[3] and Buja et al[4])
The pathogenesis of knee OA have been linked to biomechanical and biochemical changes in the cartilage of the knee joint (e.g., inability to withstand normal mechanical stresses, limited supply of nutrients and oxygen, inadequate synthesis of extracellular matrix components, increased synthesis of tissue-destructive proteinases (matrix metalloproteinases and aggrecanases) and overall apoptosis of chondrocytes)[4-7]. Recently, synovial inflammation has also been accredited as a factor limiting knee cartilage repair. Moreover, it correlates to clinical signs of knee OA such as swelling of the knee and inflammatory pain[7,8]. It is believed that synovial inflammation is a response of synovial macrophages to cartilage debris and catabolic mediators entering the synovial cavity[8,9].
In regards to the epidemiology of knee OA (Table (Table1),1), studies indicate that knee osteoarthritis in men aged 60 to 64 is usually found in the right knee (23%) than in the left knee (16.3%), while distribution seems to be more evenly balanced in women of the same age (right knee, 24.2%; left knee, 24.7%)[6,10]. A variety of endogenous (e.g., age, sex) and exogenous (obesity, patient’s lifestyle) risk factors for OA have also been outlined[2,6,11-14]. Recently, a number of genome wide association studies (GWAS) (e.g., Rotterdam GWAS[15], Tokyo GWAS[15], Chingford Study[16]) have highlighted the significance of gene mutations (e.g., in GDF5) for the development of knee OA[15-21]. Additionally, ross-sectional studies indicate that the risk of knee OA is 1.9 to 13.0 times higher among underground coal miners when compared to a control population; presumably, due to frequent work in the kneeling or squatting position[6]. Construction workers, especially floorers, also have a significantly elevated prevalence of knee OA[6].
Table 1: Worldwide prevalence (2005) of knee osteoarthritis
Knee OA Prevalence
Asia
Middle East 17.6%
South 0. 15.6%
East 0.16.8%
Southeast 0.17.0%
Central 18.5%
Pacific 17.0%
Africa
North 17.6%
West Sub-Saharan 15.7%
East Sub-Saharan 15.4%
Central Sub-Saharan 15.3%
South 18.2%
Europe
Western 16.9%
Central 18.9%
Eastern 19.1%
South America
Andean 17.5%
Tropical 16.9%
Southern 16.9%
Australia and Oceania
Australia 17.36
Oceania 18.1%
North America
United States and Canada 17.9%
Central America
Caribbean 17.6%
Combined value for male and female, aged 30-100 (Data adapted from March et al[10]). OA: Osteoarthritis.
As of clinical diagnosis of knee OA, it is complex as during the physical examination of the patient it is needed to confirm and characterise joint involvement, as well as to exclude pain and functional syndromes linked to other causes (e.g., inflammatory arthritis or damaged meniscus)[3,11,22]. In addition to non-surgical treatments for this condition such as physiotherapy, diet rich in vitamin D and supportive sport (e.g., swimming)[10,23,24], there are several medicinal and homeopathic products on the market, which promise pain relief and a decrease in symptoms. However, researchers are keen to investigate new treatments to combat OA of the knee.
Stem Cell Treatment
Self-regeneration of the cartilage, which includes chondrocytes, ground substance (cartilage matrix) and elastin fibers, is a slow process which results in new cartilage substance that is not stable for intensive burdens. The fluid inside the joint contains mesenchymal stem cells (MSCs) which can differentiate into chondrocytes, but new deposited cartilage is very fragile and can be destroyed by applying a minimal amount of stress on the joint. Additionally there is only a limited quantity of MSCs in the joint available to differentiate and the process of differentiation is slow[1,25].
Stem Cell Management
The aim in using stem cells is to support the self-healing process of the knee joint cartilage which results in relief from OA symptoms[26-32]. This treatment should be used in conjunction with additional treatment in order to improve patients’ functional status and quality of life. However, osteoarthritis cannot be cured by any radical treatment at the moment.
The stem cell candidates for use in these therapies are multipotent adult MSCs, because they are available in several tissues, including in the fluid inside the joint, and have the ability to differentiate into cells of the chondrogenic lineage[33,34]. Pittenger et al[35] have described that MSCs could be cultured without losing their multilineage differentiation potential and it has been shown that MSCs are capable of undergoing chondrogenic differentiation both in-vitro and in-vivo. MSCs can be harvested from bone marrow, periosteum, trabecular bone, adipose tissue, synovium, skeletal muscle and deciduous teeth[36]. Regardless of their origin they have the capacity to differentiate into many cell types, including cells of connective tissue lineages, including bone, fat, cartilage and muscle[26,37]. MSCs were first identified in the pioneering studies of Friedenstein and Petrakova (1966)[33] and are of major interest of research in the treatment of arthritis, in particular OA.
Multipotent adult mesenchymal stem cells are extensively investigated – in particular their behaviour in cell culture: how do they stay multipotent after several passages; how is chondrogenesis triggered in MSCs[32]. There are no definitive markers identified for MSCs yet, but the immunophenotype is positive for the proteins and enzymes STRO-1, CD73, CD146, CD105, CD106, CD166 and negative for CD11b, CD45, CD34, CD31 and CD117. These are the most reliable for characterizing MSCs[34,36].
There are several other criteria which must be considered when growing MSCs in culture. One of the most crucial criteria is the availability of characterized factors which stimulate the anabolic activity in cartilage including transforming growth factor (TGF)-β, bone morphogenetic protein (BMP), fibroblast growth factors (FGF), insulin growth factor (IGF)-1, hedgehog (hh) and Wingless (Wnt) proteins[26]. These factors are signalling proteins that belong to the tyrosine kinase family of proteins (transmembrane proteins) that activate several downstream processes leading to cell proliferation, survival, growth and a reduction in apoptotic signalling.
Growth factors like FGF2 or transforming growth factor beta induce a positive differentiation of MSCs[38]. Moreover, the development of methods was required to develop the cartilage phenotype without hypertrophy, fibrinogenesis or ossification. In addition, a delivery system was devised to target cells in a lesion, but without inhibiting their chondrogenic differentiation or the integrity of repaired tissue[39].
Clinical Trials
In recent years several clinical protocols for MSCs have been tested[26-32,40]. In general, MSC related therapeutic approaches have a significant advantage to traditional surgical approaches such as autologous chondrocyte transplantation: no cartilage biopsy is necessary, thus no external stress and cellular damage are applied at the donor-site articular surface[31]. Moreover, direct intra-articular injection of MSC is perceived as a technically simple way to treat advanced OA of the knee[32].
Stem cells from patients
MSCs and platelet-rich plasma are harvested from the patient to be treated thus ensuring that the patient’s immune system will not reject the cells[41]. These cells are already specific for the patient’s body but they have to be processed before intra-articular injection in the knee joint. This process includes separation of the MSCs by centrifugation and other purification steps. With the aim in mind of increasing cartilage build-up, chondrogenic activity of the harvested cells has to be evaluated, as well as glycosaminoglycan and type II collagen deposition, before reinjection[29]. The MSCs are tested in vitro for their ability to undergo chondrogenic differentiation under the previous described conditions. Glycosaminoglycan and type II collagen are components of the matrix of cartilage which induces and supports the differentiation of MSCs into chondrocytes. During this procedure it is important that the joint is stressed as little as possible because the newly differentiated cartilage is highly susceptible to damage.
In regards to recent advancements in the field, Neporent[42] mentioned several pro and contra factors for stem cell injection in the knee joint. MSCs treatment offers the significant advantage of a quick and relatively uneventful recovery. Furthermore the majority of patients became ambulatory within 24 h. There are no reasonable arguments against treatment with the patient’s stem cells, but there are several issues that have to be considered that are likely to make it financially less attractive. Firstly, at approximately $4000 per knee for stem cell reinjection, which will not be covered by health insurance, this treatment is not for affordable by everyone. Secondly, there are several criteria for eligibility for treatment of osteoarthritis with stem cells preparations. For one thing, the body-mass-index (BMI) should not be more than 35. Obesity, as previously mentioned, is a high risk factor for OA, because of the high stress which results on the knee joint. Stem cell treatment is reasonable, if it can be ensured that there would be no high stress on the joint. Furthermore this treatment is applicable only if the degeneration of the cartilage is not complete. As long as cartilage and joint fluid is available, stem cells can differentiate, because of necessary factors are present in the fluid and matrix but in severe cases, with bone-bone contact, stem cell treatment is unlikely to work. Most important for the patient is to minimize physical activity in the immediate period after the therapy because the stress to the joint reduces the chance of successful recovery. Furthermore it is likely that more than one treatment session would be required, meaning a greater investment of time and money.
In addition to the intra-articular injection of MSCs, Nöth et al[32] also highlighted the use of MSCs as progenitor cells to engineer cartilage implants that can be used to repair chondral and osteochondral lesions, or as trophic producers of bioactive factors to initiate endogenous regenerative activities in the OA joint.
Stem cells from donors
Another potential source of stem cells, which can be used in therapies, is allogeneic MSCs. They are harvested from donated human umbilical cord tissue (HUCT) after normal, healthy births where the mother has been tested for infectious diseases and has a screened medical history. These harvested MSCs are then screened to International Blood Bank Standards (Stem Cell Institute, 2012).
Umbilical cord tissue provides an abundant supply of mesenchymal stem cells avoiding the requirement to harvest stem cells by invasive procedures such as liposuction or bone marrow aspiration. There is evidence showing that mesenchymal stem cells from umbilical cords are more robust than those from other sources such as fat[43].
Rush University Medical Center[44], 2013, described the preparation of MSCs harvested from donated umbilical cord tissue: The cells are mixed with hyaluronan, a natural polymer that plays an important role in wound healing and deposition of cartilage, and are subsequently re-injected into the knee joint. In addition they also described a two-year Phase I/IIa clinical study in which a total of 12 participants aged 18 years and older, with a body mass index of less than 35 were enrolled. Initially, six individuals with lesions sized 2 to 5 cm were recruited into the study and an additional six volunteers with lesions larger than 5 cm were enrolled subsequently. Each participant went through an eligibility screening followed by a 12-mo observation period to determine the safety and efficacy of the therapy with an additional long-term follow-up evaluation at 24 mo.
Basically both treatment protocols, both for the MSCs from the patient and from a donor, were identical. Any differences in the MSCs and in some characteristics of the cells arose due to those from the patient themselves, from fat or bone marrow, being “older” than MSCs from umbilical cord and may therefore lack potential for proliferation and/or differentiation.
Conclusion
In recent years the role of stem cells in health and disease is a topic of high interest for biomedical research, especially regenerative medicine[33,45,46], including non-pharmacologic treatment of knee OA[25,40,47], and drug discovery[48-50]. At the moment there is an increase in the number of clinical cases utilizing cellular therapy for knee OA, however, many clinical protocols are still under development[26,30,40].
Future perspectives about clinical trials with stem cells from patients
Based on the current status of clinical investigations regarding autologous cellular therapy for OA of the knee some authors have expressed concerns about the issues of dosing , timing of intervention, type of MSCs, mode and route of delivery of MSCs in clinical studies[51-56]. Therefore the need for a gold standard for autologous cellular therapy for knee OA arises, which (hopefully) will be the aim of future clinical trials. Another interesting trend is the increased research interest in scaffold assisted or scaffoldless grafts of MSCs as a method to restore the structural and biomechanical characteristics of the OA affected knee[57-62]. MSC grafts may even prove to be a viable alternative to total knee replacement in the near future. However, we still have to wait for a 100% effective and also low cost clinical procedure to be developed.
Future perspectives about clinical trials with stem cells from donors
The use of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in clinical trials for treatment of knee OA faces the same challenges as clinical trials with other types of MSC in terms of stem cell handling[43]. There is also the need for more relevant clinical data, so it would be beneficial to have more clinical trials for knee OA, which utilize hUC-MSCs.
Future perspectives about basic research in knee cartilage regeneration and chondrogenesis in vitro and in vivo
Nowadays basic research in chondrogenesis in vitro and in vivo is primarily focus on increasing the efficacy of stem cells in terms of tissue repair[57-62]. However, the issues of stem cell characterization and tumorigenesis in vivo are somewhat overlooked.
Until relatively recently, the genomic profile of the stem cell lines maintained in vitro was only assessed in terms of ploidy and karyotype, as it was known that cultured cells may exhibit loss or gain of chromosome fragments or whole chromosomes and/or genomic rearrangements[63-65]. After the introduction of the concept for individual capacity for DNA repair and for maintenance of genomic integrity in research and diagnostic practice, its applicability as a complex marker for the proliferative potential and/or the differentiation capacity of undifferentiated cells has been extensively discussed[66-69]. Some authors have advised that the minimal panel for characterisation of in vitro maintained pluripotent cell lines ought to include markers for individual capacity for repair of genotoxic damage and maintenance of genomic integrity[69-71]. Some stem cells types (mesenchymal stem cells, haematopoietic cells from bone marrow and iPSC) have been shown to lose TP53 gene copies during in vitro culturing (detected as loss of heterozygocity for markers at the TP53 locus)[72]. Shetzer et al[72] also reported that the cells with loss of heterozygocity were more often than not identified as the origin of the teratoma-like tumours developing after the cells were transplanted in mice.
All those findings in basic stem cell biology will likely influence the development of more advanced (in terms of cell characterization) stem cell culturing and differentiation protocols and lead to the development of a gold standard in clinical trials with MSCs.
Conclusion
In conclusion, cellular therapy may not become a standard treatment for knee OA till the end of the decade due to various aspects regarding the clinical safety (e.g., risk of complications after surgery, compatibility of donor stem cells) and the affordability of this treatment for the general public. Moreover, there is still no sufficient amount of clinical data on the effectiveness of cellular therapy when compared with pharmacological treatments for this particular disease[47]. There is also the emerging application of nutraceuticals as a possible alternative to drugs for knee osteoarthritis[73,74]. So here comes the question: what will future clinical trials for knee OA and OA in general evaluate: novel pharmaceuticals, novel nutraceuticals, improved stem cell therapies?
Footnotes
P- Reviewer: Chen YK, Fenichel I, Yao YC, Zhai G S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
References
- Gupta PK, Das AK, Chullikana A, Majumdar AS. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 2012;3:25. [PMC free article] [PubMed] [Google Scholar]
- Chaganti RK, Lane NE. Risk factors for incident osteoarthritis of the hip and knee. Curr Rev Musculoskelet Med. 2011;4:99–104. [PMC free article] [PubMed] [Google Scholar]
- Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. [PubMed] [Google Scholar]
- Buja LM, Krüger GRF. Netter‘s Illustrated Human Pathology. 2nd ed. Suite, PA: Elsevier Inc; 2014. p. 390. [Google Scholar]
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. [PubMed] [Google Scholar]
- Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152–162. [PMC free article] [PubMed] [Google Scholar]
- Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–49. [PubMed] [Google Scholar]
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. [PubMed] [Google Scholar]
- Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–56. [PubMed] [Google Scholar]
- March L, Hoy D, Smith E, Blyth F, Cross M, Fransen M, Sanchez Riera L, Vos T, Buchbinder R, Brooks P, et al. Global Burden of Disease (GBD) 2010. Bone & Joint Decade 2010- Global Alliance for Musculoskeletal Health World Network Conference 2012. Cited. Suite, PA: Elsevier Inc; 2020. pp. 2014–07-20. Available from: http: //bjdonline.org/wp-content/uploads/2013/03/L-March_BJD-GLOBAL-NETWORK_Global-Burden-MSK-1990-20101.pdf. [Google Scholar]
- Pelletier JM, Pelletier JP, editors . Understanding Osteoarthritis from Bench to Bedside. Kerala, India: Research Signpost; 2011. pp. 1–26. [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. [PubMed] [Google Scholar]
- Palazzo C, Ravaud JF, Papelard A, Ravaud P, Poiraudeau S. The burden of musculoskeletal conditions. PLoS One. 2014;9:e90633. [PMC free article] [PubMed] [Google Scholar]
- Wong R, Davis AM, Badley E, Grewal R, Mohammed M. Prevalence of Arthritis and Rheumatic Diseases around the World. A Growing Burden and Implications for Health Care Needs (April 2010). Arthritis Community Research and Evaluation Unit, 2010. Cited 2014-07-22. Available from: http: //www.modelsofcare.ca/pdf/10-02.pdf.
- Loughlin J. Osteoarthritis year 2010 in review: genetics. Osteoarthritis Cartilage. 2011;19:342–345. [PubMed] [Google Scholar]
- Aref-Eshghi E, Zhang Y, Hart D, Valdes AM, Furey A, Martin G, Sun G, Rahman P, Arden N, Spector TD, et al. SMAD3 is associated with the total burden of radiographic osteoarthritis: the Chingford study. PLoS One. 2014;9:e97786. [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kondragunta V, Kornman KS, Wang HY, Duff GW, Renner JB, Jordan JM. IL-1 receptor antagonist gene as a predictive biomarker of progression of knee osteoarthritis in a population cohort. Osteoarthritis Cartilage. 2013;21:930–938. [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Spector TD. The genetic epidemiology of osteoarthritis. Curr Opin Rheumatol. 2010;22:139–143. [PubMed] [Google Scholar]
- Hochberg MC, Yerges-Armstrong L, Mitchell BD. Osteoarthritis susceptibility genes continue trickling in. Lancet. 2012;380:785–787. [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Spector TD. Genetic epidemiology of hip and knee osteoarthritis. Nat Rev Rheumatol. 2011;7:23–32. [PubMed] [Google Scholar]
- Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012;8:77–89. [PubMed] [Google Scholar]
- Paradowski PT. Osteoarthritis of the Knee: Assessing the Disease. Editorial Health Care: Current Reviews. 2014;2:e103. [Google Scholar]
- Riecke BF, Christensen R, Christensen P, Leeds AR, Boesen M, Lohmander LS, Astrup A, Bliddal H. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage. 2010;18:746–754. [PubMed] [Google Scholar]
- Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. [PMC free article] [PubMed] [Google Scholar]
- Kon E, Filardo G, Roffi A, Andriolo L, Marcacci M. New trends for knee cartilage regeneration: from cell-free scaffolds to mesenchymal stem cells. Curr Rev Musculoskelet Med. 2012;5:236–243. [PMC free article] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. [PubMed] [Google Scholar]
- Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal cellular therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14:211–215. [PubMed] [Google Scholar]
- Vinatier C, Bouffi C, Merceron C, Gordeladze J, Brondello JM, Jorgensen C, Weiss P, Guicheux J, Noël D. Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal cellular therapy. Curr Stem Cell Res Ther. 2009;4:318–329. [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. [PubMed] [Google Scholar]
- Koelling S, Miosge N. Cellular therapy for cartilage regeneration in osteoarthritis. Expert Opin Biol Ther. 2009;9:1399–1405. [PubMed] [Google Scholar]
- Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24:347–366. [PubMed] [Google Scholar]
- Nöth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–380. [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. [PubMed] [Google Scholar]
- Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. [PubMed] [Google Scholar]
- Chen FH, Rousche KT, Tuan RS. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–382. [PubMed] [Google Scholar]
- Baghaban Eslaminejad M, Malakooty Poor E. Mesenchymal stem cells as a potent cell source for articular cartilage regeneration. World J Stem Cells. 2014;6:344–354. [PMC free article] [PubMed] [Google Scholar]
- Im GI, Jung NH, Tae SK. Chondrogenic differentiation of mesenchymal stem cells isolated from patients in late adulthood: the optimal conditions of growth factors. Tissue Eng. 2006;12:527–536. [PubMed] [Google Scholar]
- Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. [PMC free article] [PubMed] [Google Scholar]
- Diekman BO, Guilak F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr Opin Rheumatol. 2013;25:119–126. [PMC free article] [PubMed] [Google Scholar]
- Wolfstadt JI, Cole BJ, Ogilvie-Harris DJ, Viswanathan S, Chahal J. Current Concepts: The Role of Mesenchymal Stem Cells in the Management of Knee Osteoarthritis. Sports Health: A Multidisciplinary Approach; 2014. [PMC free article] [PubMed] [Google Scholar]
- Neporent L Stem Cells: Alternative to Knee Replacement? Cited 2014-07-23. Available from:http: //stemcellarts.com/stem-cells-alternative-to-knee-replacement/
- Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7:195–207. [PubMed] [Google Scholar]
- Cellular therapy to repair damaged knee cartilage Rush University Medical Center. Cited: 2014-01-24. Available from : URL: http: //www.sciencedaily.com/releases/2013/01/130124163246.htm [Google Scholar]
- Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678–683. [PubMed] [Google Scholar]
- Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. [PMC free article] [PubMed] [Google Scholar]
- Singh JA. Stem cells and other innovative intra-articular therapies for osteoarthritis: what does the future hold? BMC Med. 2012;10:44. [PMC free article] [PubMed] [Google Scholar]
- Zhelev N, Trifonov D, Wang S, El Serafi I, Mitev V. From Roscovitive to CYC 202 to Seliciclib – from bench to bedside: discovery and development. BioDiscovery. 2013;10:1. [Google Scholar]
- Zhelev N, Tummala H. Trifonov D., D’ Ascanio I., Oluwaseun O.A., Fischer P.M. Recent advances in the development of cyclin-dependent kinase inhibitors as new therapeutics in oncology and cardiology. Curr Opin Biotech. 2013;24:25. [Google Scholar]
- Trifonov D, Tummala H. Clements S., Zhelev N. Effect of roscovitine on cardiac hypertrophy in human stem cell derived cardiomyocytes. Curr Opin Biotech. 2013;24:114. [Google Scholar]
- Viswanathan S, Gómez-Aristizábal A. Review of Patents and Commercial Opportunities Involving Mesenchymal Stromal Cells (MSCs) Therapies in Osteoarthritis. Recent Patents on Regenerative Medicine. 2014;4:1–15. [Google Scholar]
- Wei CC, Lin AB, Hung SC. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant. 2014;23:505–512. [PubMed] [Google Scholar]
- Jorgensen C, Noël D. Mesenchymal stem cells in osteoarticular diseases: an update. Cited 2014-07-26. Available from: http: //www.ijmcmed.org/files/site1/user_files_a195ea/eng/jorgensen-A-10-37-1-8b83de9.pdf.
- Guérit D, Maumus M, Apparailly F, Jorgensen C, Noël D. Therapeutic mesenchymal stem or stromal cells in rheumatic diseases: rationale, clinical data and perspectives. J Clin Invest. 2011;1:1269–1277. [Google Scholar]
- Wang W, Cao W. Treatment of osteoarthritis with mesenchymal stem cells. Sci China Life Sci. 2014;57:586–595. [PubMed] [Google Scholar]
- Montoya F, Martínez F, García-Robles M, Balmaceda-Aguilera C, Koch X, Rodríguez F, Silva-Álvarez C, Salazar K, Ulloa V, Nualart F. Clinical and experimental approaches to knee cartilage lesion repair and mesenchymal stem cell chondrocyte differentiation. Biol Res. 2013;46:441–451. [PubMed] [Google Scholar]
- Hollander AP, Dickinson SC, Kafienah W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cells. 2010;28:1992–1996. [PMC free article] [PubMed] [Google Scholar]
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. [PMC free article] [PubMed] [Google Scholar]
- Jakobsen RB, Shahdadfar A, Reinholt FP, Brinchmann JE. Chondrogenesis in a hyaluronic acid scaffold: comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg Sports Traumatol Arthrosc. 2010;18:1407–1416. [PubMed] [Google Scholar]
- Orth P, Rey-Rico A, Venkatesan JK, Madry H, Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning. 2014;7:1–17. [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, et al. Tissue engineering for articular cartilage repair–the state of the art. Eur Cell Mater. 2013;25:248–267. [PubMed] [Google Scholar]
- Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J Orthop. 2014;5:80–88. [PMC free article] [PubMed] [Google Scholar]
- Arabadjiev A, Petkova R, Momchilova A, Chakarov S, Pankov R. Of mice and men – differential mechanisms of maintaining the undifferentiated state in mESC and hESC. Biodiscovery. 2012;3:1. [Google Scholar]
- Lefort N, Feyeux M, Bas C, Féraud O, Bennaceur-Griscelli A, Tachdjian G, Peschanski M, Perrier AL. Human embryonic stem cells reveal recurrent genomic instability at 20q11.21. Nat Biotechnol. 2008;26:1364–1366. [PubMed] [Google Scholar]
- Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol. 2008;26:1361–1363. [PubMed] [Google Scholar]
- Hyka-Nouspikel N, Desmarais J, Gokhale PJ, Jones M, Meuth M, Andrews PW, Nouspikel T. Deficient DNA damage response and cell cycle checkpoints lead to accumulation of point mutations in human embryonic stem cells. Stem Cells. 2012;30:1901–1910. [PubMed] [Google Scholar]
- Petkova R, Chelenkova P, Georgieva E, Chakarov St. What’s your poison? Impact of individual repair capacity on the outcomes of genotoxic therapies in cancer. Part I – role of individual repair capacity in the constitution of risk for late-onset multifactorial disease. Biotechnol Biotec Eq. 2013;27:4208–4216. [PMC free article] [PubMed] [Google Scholar]
- Lund RJ, Närvä E, Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nat Rev Genet. 2012;13:732–744. [PubMed] [Google Scholar]
- Rocha CR, Lerner LK, Okamoto OK, Marchetto MC, Menck CF. The role of DNA repair in the pluripotency and differentiation of human stem cells. Mutat Res. 2013;752:25–35. [PubMed] [Google Scholar]
- Chelenkova P, Petkova R, D’ Ascanio I, Zhelev N, Chakarov S. In sickness and in health: a set of markers for individual repair capacity in risk assessment, monitoring and prognosis of human disease. Curr Opin Biotech. 2013;24:105. [Google Scholar]
- Petkova R, Chelenkova P, Georgieva E, Chakarov St. What’s your poison? Impact of individual repair capacity on the outcomes of genotoxic therapies in cancer. Part II – information content and validity of biomarkers for individual repair capacity in the assessment of outcomes of anticancer therapy. Biotechnol Biotec Eq. 2014;28:2–7. [PMC free article] [PubMed] [Google Scholar]
- Shetzer Y, Kagan S, Koifman G, Sarig R, Kogan-Sakin I, Charni M, Kaufman T, Zapatka M, Molchadsky A, Rivlin N, et al. The onset of p53 loss of heterozygosity is differentially induced in various stem cell types and may involve the loss of either allele. Cell Death Differ. 2014;21:1419–1431. [PMC free article] [PubMed] [Google Scholar]
- Ragle RL, Sawitzke AD. Nutraceuticals in the management of osteoarthritis : a critical review. Drugs Aging. 2012;29:717–731. [PubMed] [Google Scholar]
- Akhtar N, Haqqi TM. Current nutraceuticals in the management of osteoarthritis: a review. Ther Adv Musculoskelet Dis. 2012;4:181–207. [PMC free article] [PubMed] [Google Scholar]
Stem cell application for osteoarthritis in the knee joint: A minireviewArticles from World Journal of Stem Cells are provided here courtesy of Baishideng Publishing Group Inc
Cellular Therapy is a successful treatment for damaged knees.
At the Hansen Clinic, we support our patients in optimizing their health through the application of advanced technologies, including stem cell therapy. We look forward to supporting you in your journey to optimal, pain-free health.
Read how Cellular Therapy helps improve rheumatoid arthritis.
Ready to schedule your Cellular Therapy consultation?


